Purpose : To control non-conforming product and to continually improve the management system
Scope:Applies to all activities.
Owner : QMR
Approver:
MD
Date:
Revision Number:

Non-Conforming Product (Supplied)
Non-conforming
Material Identified.
Quarantine Item

Non-Conformance
raised by QC
Department and
Supplier Informed

Decision required for
product -
Rework,Disposition
from Client or Reject
and noted on NCR.




Process Number: QP13 - Non Conforming Product, Corrective And Preventive Action & Customer Compliant

Non-Conforming Product (Internal)
Non-conforming
Material Identified.
Quarantine Item
and Inform QC
Department

Non-Conformance
raised by QC
Department. No
further process
carried out on item.

Decision required for
product -
Rework,Disposition
from Client or Reject
and noted on NCR.





Corrective and Preventive Action
Corrective Action: -
Action taken to
eliminate the cause
of a detected non-
conformity.

Preventive Action -
Action taken to
eliminate the cause
of a potential non-
conformity.

All Non-
conformances are
investigated to
identify root cause
and identify actions.

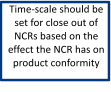




Customer Complaint
Customer Complaint
Received. MD
informed and
acknowledgement
sent to Customer.