


Purpose : To ensure that document required by the QMS are created, reviewed before use, regularly reviewed and updated and the distribution controlled.
Scope:Applies to all QMS documents
Owner : Q.M.R
Approver: Director
Date:
Revision Number:

New Document
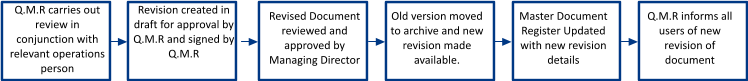
Periodic Review

Process Number: QP03 - Document Control
External Documents


















![Close [x]](index_htm_files/close.png)